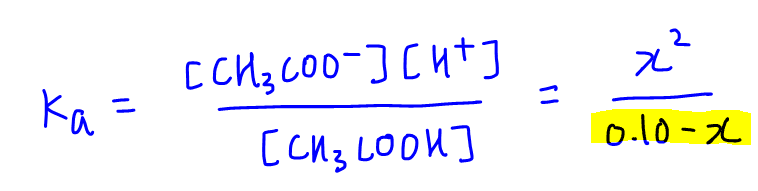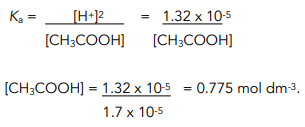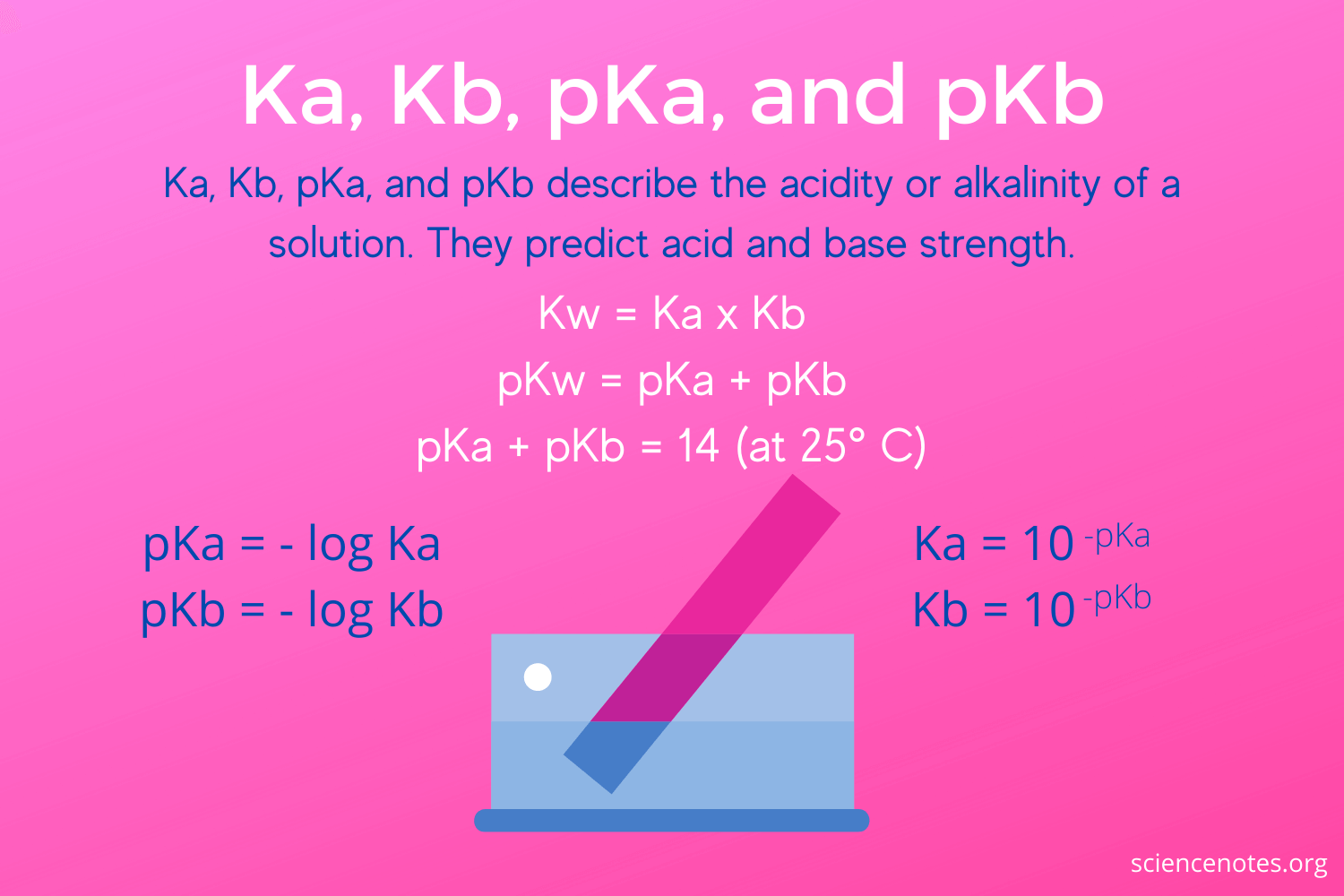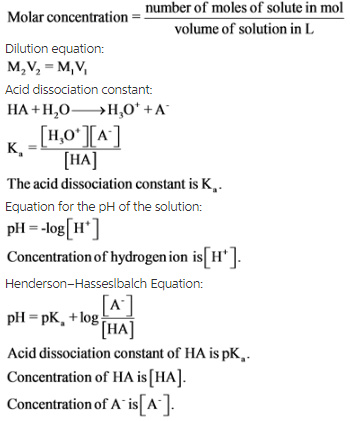
The Ka value for acetic acid, CH3COOH(aq), is 1.8x10^-5. Calculate the ph of a 2.80 M acetic acid solution - Home Work Help - Learn CBSE Forum
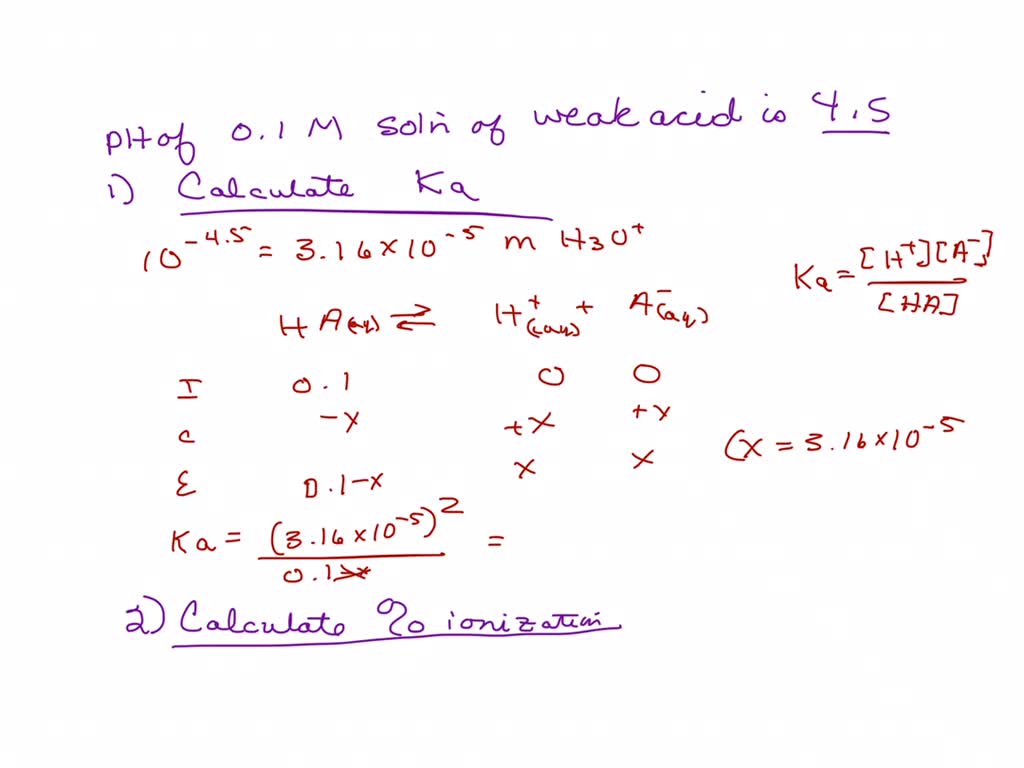
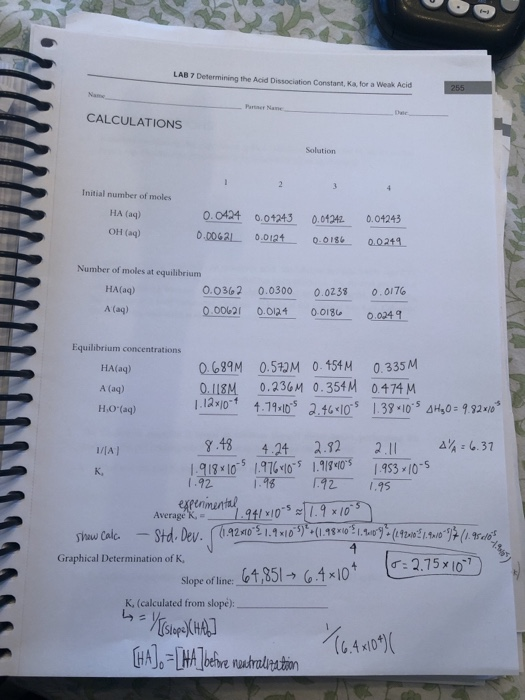
SOLVED: CHEM 1312 NAME The pH of a 0.1 M solution of the weak acid HA is 4.5. Calculate the Ka for the acid. (5 POINTS) b. Calculate the percent ionization for the acid. (3 POINTS)

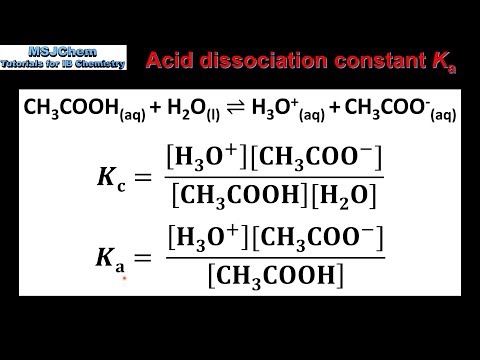
How to calculate Ka from pKa? - pka to ka, Conversion, Examples | Conjugate acid, Relatable, Dissociation